
By Sarah Paris
In the world of research, precision medicine is advancing at a breakneck speed. Scientists are creating algorithms to help predict outcomes: what type of patient, for example, will respond best to a certain drug used in the treatment of COVID-19? But once those algorithms exist, how can they be applied to an individual patient in the clinic? How can the data be extracted and presented as a picture that informs clinical decision-making?
A UCSF team has developed a new tool they hope will bridge this divide.
In her clinical research lab, UCSF Professor of Neurology Kate Rankin, PhD, has a plethora of high-tech tools at her disposal to analyze, process, and compare patient data relevant to her research. But until recently, that access evaporated as soon as she walked across the street and into her clinic. There, she was obliged to pull out an old-fashioned pocket calculator and a reference book just to calculate the cognitive score of a patient, based on the questionnaire they had filled out in the waiting room.
Rankin was not alone in her frustration over this technological mismatch. Other clinician-scientists at the UCSF Weill Institute for Neurosciences were questioning the status quo as well. They decided to come together to address the issue collaboratively. The team included:
- Riley Bove, MD MMSc, the clinical lead for the MS Bioscreen, who had designed several platforms to pull together, process and visualize a patient’s clinical and research data, both within UCSF and with collaborators at Sutter (NeuroShare);
- Stephan Sanders, BMBS PhD, a computational geneticist, who directs the Psychiatry Department Bioinformatics Core, which designs and implements cloud-based genomic analysis for the UCSF community;
- Kate Rankin, PhD, who is a leader in UCSF’s precision medicine initiative and had previously built a tool called Brainsight to automate complex processing of neuroimaging for research patients.

Precision Medicine and COVID-19
The BRIDGE team is currently working on building a new dashboard that would help non-specialist physicians work with individuals with acute respiratory symptoms to help manage their conditions
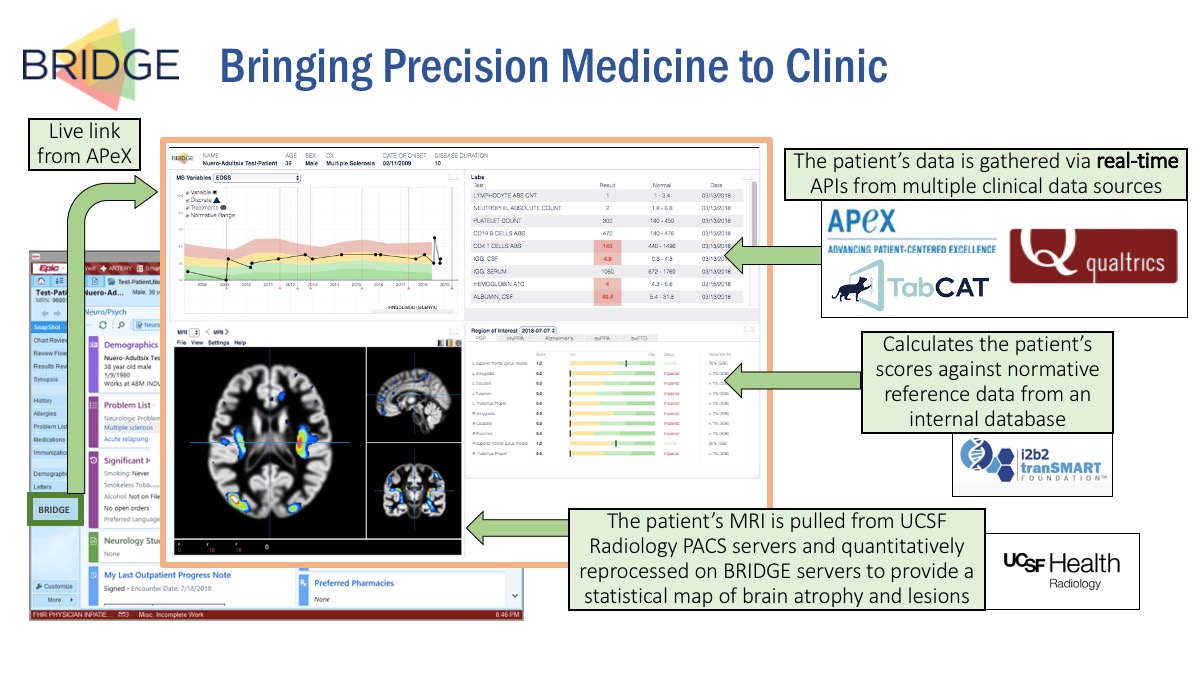
The three researchers engaged software engineer Erica Schleimer to lead the structural development of the new tool aptly called “BRIDGE.” Over three years in the making, BRIDGE has now been successfully piloted in a number of clinics and is on track to be rolled out to more. Importantly, the tool has passed the regulatory process that was needed to connect it to the Electronic Health Record (EHR).
“Our goal with BRIDGE was to resolve the technical obstacles that keep clinicians from seeing the information they want during clinical encounters. The clinicians themselves are the best judge of how that data should be organized, quantified, and displayed to help them provide better care. BRIDGE just makes that possible, in real time, with real patients," said Rankin.
Essentially, BRIDGE is an interface available to physicians during their encounter with patients. As they consult the EHR on their computer screen, they can click the BRIDGE link, and the platform opens up. BRIDGE then pulls that patient’s data from the EHR, as well as from many other sources, including images from radiology, data from questionnaires, and results from cognitive tests the patient performed earlier.
BRIDGE runs these data through established quantifications and algorithms, chosen by clinics based on their unique diagnostic needs. The data are shown through visual graphics for quicker interpretation. This makes it far easier for physicians to draw evidence-based conclusions about diagnosis, treatment and prognosis of their patient.
BRIDGE also makes it feasible to share meaningful data with patients, showing, for example, how their cognitive score has improved over time. Graphs and visualizations that clearly indicate improvement or progression can help patients understand their health journey and create a basis for shared decision-making.
DATA BECOMES KNOWLEDGE
The EHR has made it possible to collect enormous amounts of data. But a typical EHR interface is filled with numbers and tables that can be hard to piece together into a coherent story, even for clinicians. While some visualizations are being developed by EPIC (the dominant EHR platform, known at UCSF as APeX), those are meant to serve a broad range of users and clinics. Customization to focus on a specific disease is not something EPIC can or likely will provide. Moreover, changes to EPIC require many months, if not years. And EPIC cannot accommodate complex machine learning of the type that will be required by precision medicine. This is the gap BRIDGE aims to fill.
“From the start, we designed BRIDGE to be modular," said Bove. "It takes the form of a dashboard that can be readily customized and adapted to the needs of additional clinics both within and outside the Weill Institute of Neurosciences. The modular components reduce the barrier to entry for any new clinic, so that individual groups do not have to reinvent technological or institutional processes. Once clinical researchers specify which data types and visualizations are most relevant to their patients, they can then focus on their domain expertise."
The team is engaging in discussions with clinics in departments outside of Neurology and Psychiatry and working on adapting the tool in different ways.
Thus far, the work has been supported by grants and philanthropy, in particular, a generous Weill Innovation Award. It currently falls into the category of a clinical research application. To scale it up beyond its initial limited scope into broader clinical care would require not only more funding, but a wider discussion on how this tool might fit into the larger digital roadmap for UCSF.
The potential gain is immense: BRIDGE is able to to integrate a number of deep data sources beyond the EHR, such as imaging and genomics data. The jackpot for one recently initiated project, supported by the George and Judy Marcus Program for Precision Medicine Innovation, will be the fully implemented connection of BRIDGE to two other precision medicine treasure troves:
- the Information Commons, a repository of UCSF basic, clinical and population data
- SPOKE, a massive “database of databases” that computationally merges ~30 publicly available data sources.
Another exciting project in progress is the integration of mobile health and patient-generated data into BRIDGE. Some worry that this flood of information could be overwhelming for clinicians already strapped for time. But the BRIDGE investigators believe that a well-constructed dashboard will actually focus the flow of relevant information in ways that will enable faster and better decision-making.
“My bias has always been to make data relevant and helpful,” Rankin said. “Often, physicians are in a quandary, trying to decide between two diagnoses. This tool could help clarify decision-making. Does this patient’s profile look more like possible diagnosis A or B?”
Another potential use of BRIDGE is in the training of students and residents. Consulting the BRIDGE interface as part of the diagnostic journey could highlight an easily overlooked symptom or suggest a non-standard medication.
In each of these instances, BRIDGE serves as a paradigm of how precision medicine can manifest in the clinical setting.
“BRIDGE is a spectacular tool that connects multiple data sources and delivers actionable visualizations to the physician and the patient,” said Keith Yamamoto, PhD, director of UCSF Precision Medicine and of the Marcus Program for Precision Medicine Innovation. “Linkage of BRIDGE to SPOKE and the Information Commons will enable knowledge from basic and population research to be integrated into patient clinical data in real time—a principal mission of precision medicine.”
